The Ideal Breast Implant
While the silicone gel implant has a certain appeal, many women are uncomfortable with the idea they could have a silent rupture, undetectable except with an MRI scan, and prefer to have saline instead of silicone gel in their body. Women have expressed their desire for an improved saline-filled breast implant, one that gives a natural appearance and tissue-like feel similar to the silicone gel implant, but not the wrinkling, bouncing and globular look of the current saline implant. In short, a “hybrid” breast implant.
Ideal Implant Incorporated was founded January 2006 to develop an ideal breast implant that would combine the best of both current implants: the natural result of silicone gel and the safety of saline for “Peace of Mind.”
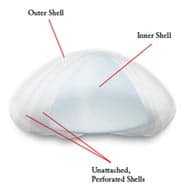
 Years of research, testing and input from women and plastic surgeons led to the design of the investigational IDEAL IMPLANT® Saline-filled Breast Implant. On the outside, it looks like a standard saline implant, except that the edges have been lowered so it may contour better to the chest wall. On the inside, it simply contains a series of additional implant shells that are nested together. This internal structure was designed for control of saline movement to reduce bouncing, and for support of the implant edges to minimize wrinkling and prevent collapse of the upper portion of the implant.
Years of research, testing and input from women and plastic surgeons led to the design of the investigational IDEAL IMPLANT® Saline-filled Breast Implant. On the outside, it looks like a standard saline implant, except that the edges have been lowered so it may contour better to the chest wall. On the inside, it simply contains a series of additional implant shells that are nested together. This internal structure was designed for control of saline movement to reduce bouncing, and for support of the implant edges to minimize wrinkling and prevent collapse of the upper portion of the implant.
The Clinical Trial
The IDEAL IMPLANT® is now being studied in an FDA-approved nationwide clinical trial, limited to 500 women having their first breast augmentation or replacement of existing saline or silicone gel implants. Donald W. Hause, M.D., is an investigator and has the information about how you can become a participant in this study.
The Ideal Implant Study Participants’ Trust Fund
Payment for Participation
You will not receive any payment for enrolling in this study. All costs of your surgical procedure are your responsibility.
You will receive payment for participation in the follow-up visits over 10 years that give information on safety, efficacy and outcome of the study implants.
If you complete ALL required follow-up visits during the 10-year follow-up visit phase of the study, you will receive a lump sum payment from an independent Trust Fund. Required follow-up visits are at 2 and 6 months, and at 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 years.
In addition, the Trust Fund will act as a financial protection plan to reduce your financial risk should Ideal Implant Incorporated cease operations. In that case, you could use your Trust Fund payment toward the cost of removal or replacement of your study implants.
As a leading Sacramento breast augmentation surgeon, Dr. Hause is at the forefront of emerging technologies and techniques. His involvement in the Ideal Breast Implant clinical study is an example of his dedication to evolving the plastic surgery field. From this state-of-the-art facility, he has treated numerous patients seeking plastic surgery in Sacramento.
